RARE DISEASE POLICIES IN EUROPE
Description of the report
From 2012-2015, the ‘State of the Art’ Report was published annually, in 5 volumes, under the EUCERD Joint Action (past volumes are available here)
In 2016, the Resource on the State of the Art of Rare Disease Activities in Europe migrated to RD-ACTION.
The streamlined State of the Art Resource comprises the following components:
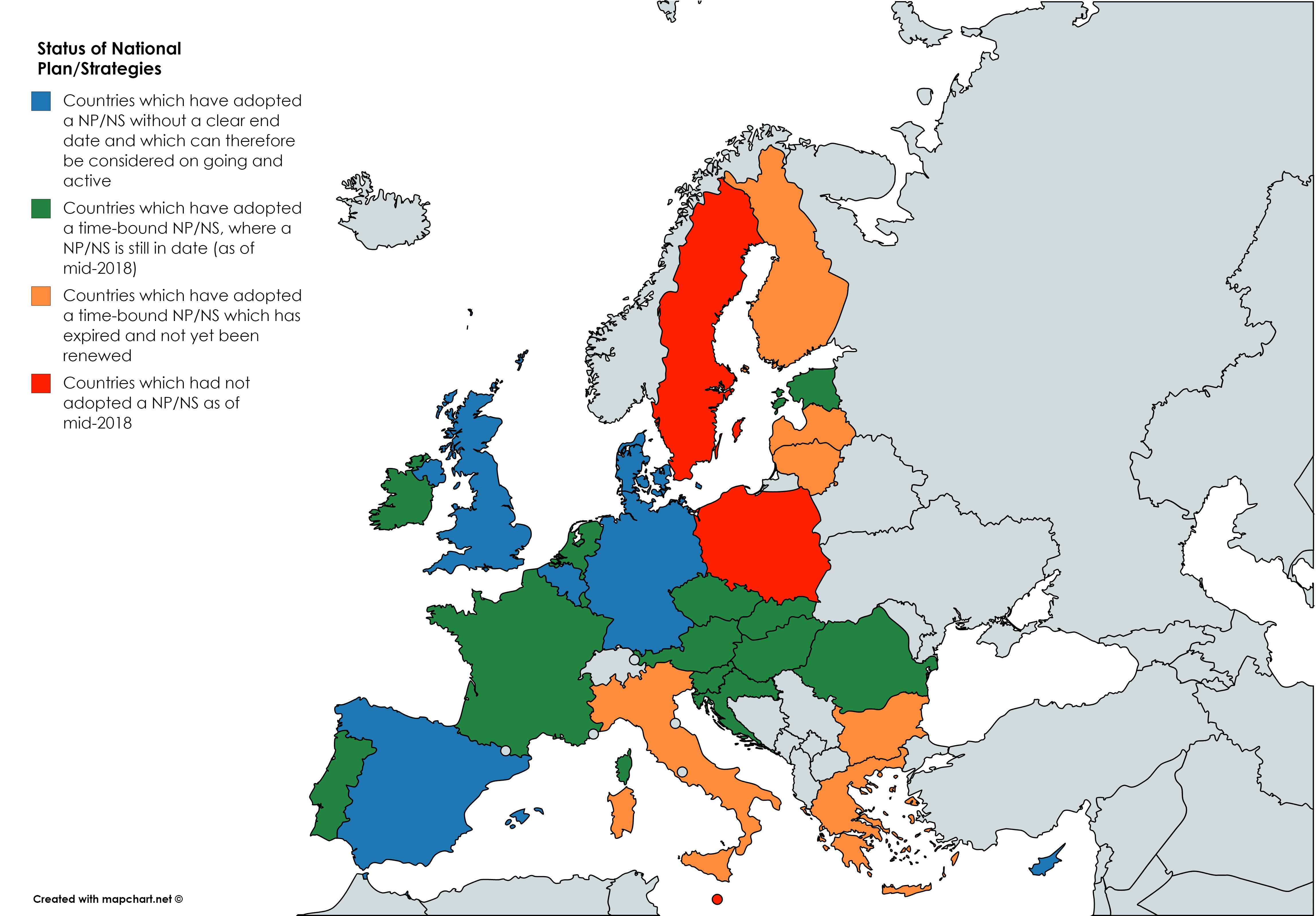
- An Overview Report (summarizing key European RD policy-related watersheds and documents; the status quo in Europe regarding national plans and strategies; highlights on transversal topics such as registries, genetic testing, research; summaries of the RD policy frameworks of non-European countries (based upon the OrphaNews newsletter; highlights of RD institutions such as Orphanet and EURORDIS; and more)
- Country-specific data relating to rare disease activities at the national level (including summaries, more detailed reports, and archived information)
- Technical Guidance for completing the survey is available SotaR Survey Technical Guidance 2019 Update:
- The full survey -showing the question maps and possible replies under each section and sub-section- is available here: Final State of the Art question bank
- Topic Summaries will be elaborated under the Rare2030 project on issues including the following: the Status Quo of National Plans and Strategies for Rare Diseases in Europe; Newborn Screening; Centres of Expertise; and rare disease Registries. Once the last remaining countries have submitted their data completed table of EUCERD Core Indicators for National Plans/Strategies will be published here
The Country-Specific Data will continue to be collected after the end of the RD-ACTION funding period, led by Newcastle University and supported (partially) by Rare2030 (https://www.rare2030.eu/)